-
PDF
- Split View
-
Views
-
Cite
Cite
Shuixia Guo, Lena Palaniyappan, Bo Yang, Zhening Liu, Zhimin Xue, Jianfeng Feng, Anatomical Distance Affects Functional Connectivity in Patients With Schizophrenia and Their Siblings, Schizophrenia Bulletin, Volume 40, Issue 2, March 2014, Pages 449–459, https://doi.org/10.1093/schbul/sbt163
Close - Share Icon Share
Abstract
Background: The efficiency of human brain depends on the integrity of both long- and short-range connections, but the long-range connections need to be “penalized” to reduce overall wiring costs. This principle, termed as the anatomical distance function (ADF), refers to the presence of an inverse relationship between anatomical distance and connectivity. A crucial developmental feature that occurs in normal adolescence is the weakening of ADF, which is characterized by a selective strengthening of long-distance connections. Schizophrenia is associated with widespread dysconnectivity that is linked to aberrant cortical development. Methods: We studied the ADF in adults with schizophrenia (n = 28), their age-matched siblings (n = 28), and healthy controls (n = 60). We investigated the proportional abnormalities in the long-range connections involving interhemispheric, subcortical, frontal, and salience network regions and localized the connections showing most significant changes in schizophrenia. The groups were discriminated on the basis of short- and long-range connectivity using a machine-learning algorithm. Results: Both patients and their siblings showed abnormally pronounced ADF. This was associated with a disproportionate reduction in the number of long-range connections, affecting the subcortical, interhemispheric, and the salience network connections. The abnormalities in long-range connections had superior ability to accurately identify group membership. Conclusions: A crucial organizing principle of the brain architecture that becomes apparent during normal adolescence is disturbed in schizophrenia. While siblings show some evidence of compensating for this deficit, patients lack putative compensatory changes. Age-related shift in ADF provides an explanatory framework for the developmental emergence of widespread dysconnectivity that is influenced by genetic risk in schizophrenia.
Introduction
Schizophrenia is a highly heritable disorder characterized by several features suggestive of an aberrant brain developmental trajectory.1–4 The last 2 decades of neuroscientific research has strongly supported the role of dysconnectivity in the pathophysiology of psychotic disorders such as schizophrenia. Dysconnectivity has been demonstrated both at microscale (at neuronal circuit or synaptic level)5,6 and at mesoscale (using neuroimaging measures of structural and functional connectivity) levels. A wide range of neuroimaging studies, including those investigating grey matter surface,7 anatomical covariance,8 white matter diffusion,9 macromolecular integrity,10,11 functional correlation,12,13 and temporal precedence,14,15 have been interpreted as evidence for dysconnectivity in schizophrenia. These studies have indicated that this dysconnectivity is distributed in nature, affecting the entire brain, though some regions may be affected more than the others. Bulk of evidence points towards consistent involvement of subcortical and frontal systems16 along with paralimbic salience-processing networks involving the insula, anterior cingulate cortex, and the putamen.17 Further, interhemispheric connections form a major portion of the functional connectivity in the resting brain18; aberrant interhemispheric connectivity has also been prominently observed in schizophrenia.19,20 Despite this consistency, there is a significant heterogeneity among patients in both the degree and the distribution of dysconnectivity. So far, it is unclear whether common mechanistic factors underlie the distributed pattern of dysconnectivity observed in patients. Given the criticality of adolescence in the development of psychosis,21 the mechanisms that underlie the normal development of brain as a highly connected and organized structure are strong suspects in the pathophysiology of schizophrenia.
The efficient, well-organized functioning of human brain depends on both long- and short-range connections. But the existence of long-range connections have to be limited by the spatial, material, and time-cost constraints placed on the organization of human brain.22 Long-distance connections operate at a higher metabolic cost22,23; as a result, anatomical distance is seen as a penalty for brain connectivity. In human brain functional networks, short-distance connections predominate with greater strength of functional connectivity24 while long-distance connections show reduced (or negative) connectivity strength.25 In other words, connectivity strength operates on the basis of a negative anatomical distance function (ADF). However, a connected system that indiscriminately penalizes all long-distance connections would be inefficient for communication.26 In particular, preferential long-distance connections support the emergence of highly connected brain hubs that are critical for information transfer.27 This selective permissiveness that favors long-distance connections has been observed to emerge during normal adolescent cortical development. In this period, the number of short-range connections gradually weakens with an increase in long-range connectivity.28,29 Notably, the reshuffling of the strength of positive and negative correlations among brain regions during adolescence is associated with the development of cognitive control30 and predominantly involves the insular and frontal networks.29,31 While the distance-function is a strong organizing principle for brain connectivity, a selective permissiveness of long-range connections during adolescent brain maturation appears necessary for neural efficiency of the adult brain.
The genetic diathesis to develop schizophrenia affects the normal patterns of brain connectivity. Functional magnetic resonance imaging (fMRI) studies indicate that both patients and their siblings display dysconnectivity affecting distributed brain networks.32,33 These observations indicate that shared mechanisms, possibly of genetic origins, may operate in shaping the dysconnectivity observed in adult patients and siblings. At present, it is not known whether the ADF is affected by the genetic risk of schizophrenia. The only evidence to date of a disturbed ADF in schizophrenia comes from a selected group of patients with childhood-onset schizophrenia (COS) (mean age of onset <10 years) in whom short-range connections were significantly weakened.34 At present, it is not known whether the ADF and the selective permissiveness of long-range connections are disturbed in a more representative sample of adult patients with schizophrenia and their siblings.
In the current study, using resting-state fMRI, we investigated whether the ADF is perturbed in adult patients with schizophrenia and their age-matched siblings. We hypothesized that the normal developmental weakening of the ADF noted in healthy adolescents will be deviant in patients and siblings, resulting in a reduction in long-range and increase in short-range connectivity. We expected the long-range connections involving interhemispheric, subcortical, frontal, and paralimbic salience-processing network to be disproportionately affected. In addition, we also localized the paired regional connections showing most significant changes in connectivity in patients and siblings compared with healthy controls.
Methods
Participants
Twenty-eight patients with schizophrenia, 28 sex-matched siblings free of psychiatric disorders (1 per patient), and 60 healthy controls recruited from Changsha, China were included in this study. Seven of the patients were drug naive, while the rest were receiving antipsychotic medications at the time of image acquisition. The clinical and demographic details of the sample are presented in table 1 and in the supplementary material. There was no significant difference between the 3 groups regarding sex, age, and education. All participants gave their written informed consent to participate in the study after the risks and benefits were discussed in detail. The study was approved by the ethics committee of the Second Xiangya Hospital, Central South University.
Demographic and Clinical Characteristics
| . | Patients with Schizophrenia (n = 28) . | Healthy Siblings (n = 28) . | Healthy Controls (n = 60) . | P Value . |
|---|---|---|---|---|
| Age (year) | 25.3571±5.8323 | 25.7857±6.4369 | 27.1667±6.6388 | .3978 |
| Sex (male/female) | 15/13 | 15/13 | 35/25 | .8752 |
| Education (year) | 12.3929±2.4846 | 12.5714±2.7679 | 13.5167±3.1596 | .1626 |
| Illness duration (month) | 18.32±15.84 | — | — | — |
| PANSS | ||||
| Total score | 85.9259±12.584 | — | — | — |
| Positive scale score | 21.667±4.795 | — | — | — |
| Negative scale score | 23.407±5.759 | — | — | — |
| General psychopathology scale score | 40.880±7.051 | — | — | — |
| . | Patients with Schizophrenia (n = 28) . | Healthy Siblings (n = 28) . | Healthy Controls (n = 60) . | P Value . |
|---|---|---|---|---|
| Age (year) | 25.3571±5.8323 | 25.7857±6.4369 | 27.1667±6.6388 | .3978 |
| Sex (male/female) | 15/13 | 15/13 | 35/25 | .8752 |
| Education (year) | 12.3929±2.4846 | 12.5714±2.7679 | 13.5167±3.1596 | .1626 |
| Illness duration (month) | 18.32±15.84 | — | — | — |
| PANSS | ||||
| Total score | 85.9259±12.584 | — | — | — |
| Positive scale score | 21.667±4.795 | — | — | — |
| Negative scale score | 23.407±5.759 | — | — | — |
| General psychopathology scale score | 40.880±7.051 | — | — | — |
Note: PANSS, Positive and Negative Syndrome Scale.
Demographic and Clinical Characteristics
| . | Patients with Schizophrenia (n = 28) . | Healthy Siblings (n = 28) . | Healthy Controls (n = 60) . | P Value . |
|---|---|---|---|---|
| Age (year) | 25.3571±5.8323 | 25.7857±6.4369 | 27.1667±6.6388 | .3978 |
| Sex (male/female) | 15/13 | 15/13 | 35/25 | .8752 |
| Education (year) | 12.3929±2.4846 | 12.5714±2.7679 | 13.5167±3.1596 | .1626 |
| Illness duration (month) | 18.32±15.84 | — | — | — |
| PANSS | ||||
| Total score | 85.9259±12.584 | — | — | — |
| Positive scale score | 21.667±4.795 | — | — | — |
| Negative scale score | 23.407±5.759 | — | — | — |
| General psychopathology scale score | 40.880±7.051 | — | — | — |
| . | Patients with Schizophrenia (n = 28) . | Healthy Siblings (n = 28) . | Healthy Controls (n = 60) . | P Value . |
|---|---|---|---|---|
| Age (year) | 25.3571±5.8323 | 25.7857±6.4369 | 27.1667±6.6388 | .3978 |
| Sex (male/female) | 15/13 | 15/13 | 35/25 | .8752 |
| Education (year) | 12.3929±2.4846 | 12.5714±2.7679 | 13.5167±3.1596 | .1626 |
| Illness duration (month) | 18.32±15.84 | — | — | — |
| PANSS | ||||
| Total score | 85.9259±12.584 | — | — | — |
| Positive scale score | 21.667±4.795 | — | — | — |
| Negative scale score | 23.407±5.759 | — | — | — |
| General psychopathology scale score | 40.880±7.051 | — | — | — |
Note: PANSS, Positive and Negative Syndrome Scale.
Imaging Acquisition
Eyes-closed 6-minutes resting scans were acquired on a 1.5-Tesla GE Signa Twinspeed scanner (General Electric Medical System) using a gradient-echo echo-planar sequence (repetition time/echo time [TR/TE] = 2000/40 ms, flip angle = 90°, field of view = 240 × 240 mm2). Whole-brain volumes were acquired with 20 contiguous 5-mm thick transverse slices with a 1-mm gap and 3.75 × 3.75mm2 in-plane resolution.
Data Preprocessing
fMRI data was preprocessed using SPM8 and data processing assistant for resting-state fMRI.35 Briefly, the scans were slice-timing corrected, realigned, spatially normalized to a standard template (Montreal Neurological Institute), detrended, and band-pass filtered (0.01–0.08 Hz) to reduce low-frequency drift and high-frequency physiological noise. Global noise levels were assessed to determine the suitability of global signal removal.36 Head motion parameters, global mean signals, white matter signals, and cerebrospinal signals were regressed out from the blood-oxygen-level-dependent (BOLD) signals. An automated anatomical labeling (AAL) atlas,37 as we have described in our previous article,38 was employed to parcellate the brain into 90 regions of interest (ROIs) (45 in each hemisphere). Further details on the sensitivity of preprocessing are provided in the supplementary material.
Anatomical Distance
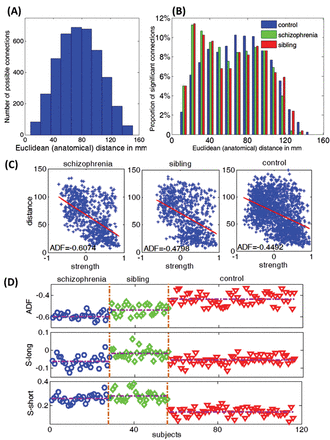
After data preprocessing, the time series were extracted in each ROI by averaging the signals of all voxels within that region. By calculating ordinary Pearson correlation coefficients between all pairs of ROIs, a 90×90 correlation matrix was obtained for all subjects. Fisher’s z transformation of these correlations provided the connectivity strength measure for each pair of regions. The anatomical distance was estimated as the Euclidean distance between the centroids of the 2 pairs of brain regions.28,34 This must be considered as the physical distance within the spatial constraints of brain rather than the actual lengths of the axonal pathways. The median distance was calculated as 75.5mm; pairs with <75.5-mm distance were classified as short-range (2001) connections and those with >75.5-mm distance were classified as long-range (2004) connections. While there is no strict definition of what a long-distance connection is, the median-split approach has provided meaningful categorization for both structural covariance34 and functional connectivity.19Figure 1A and 1B shows the distribution of the connections in the 3 groups.
We wish to print this figure with color reproduction. (A) Histogram of the anatomical distance of all possible connections. The range of the anatomical distance is 7.6~151mm. We select 75.5mm as the threshold to divide all possible connections to long-range connections (>75.5mm, 2004 connections in total) and short-range connections (<75.5mm, 2001 connections in total). (B) Histogram of the anatomical distance of all functional significant links within each group. (C) Anatomical distance function (ADF) of a single subject within each group. There are 779 significant links within group 1(schizophrenia), 780 significant links within group 2 (sibling) and 1186 links within group 3 (control). Blue stars represent these significant links; the slope of the red line is ADF of a single subject. (D) ADF, S-long and S-short for all subjects within each group. The detailed results are shown in table 2.
Statistical Analysis
For each of the 3 groups, we used a 1-sample t test at Bonferroni-corrected threshold of P < .05 to identify all functional links (positive or negative correlations) of interest within each group. For each subject, the ADF was then calculated using Fisher’s z-transformed coefficients of the linear correlation between the anatomical distance and the connectivity strength for the significant functional links in each group. The proportion of long-range connections (P-long) and the proportion of short-range connections (P-short) among all links reaching group-level significance was computed for each subject. Mean strength of all significant short-range (S-short) and long-range connections (S-long) were computed for each individual. We also calculated the proportion of all significant long-range connections that involved frontal regions, subcortical regions and the salience network. Interhemispheric connections were identified as those involving both right and left hemisphere. The proportion of long-range interhemispheric connections was also estimated for each subject. For the top 10% of significant links in each group, we computed the mean anatomical distance (MAD) to estimate the distance distribution of the most prominent connections in each group.
The 3 groups were compared with 1-way analysis of variances (ANOVA) and post hoc t tests for mean values and chi-square tests for proportions. In addition, we used support vector machines (SVMs) to discriminate the groups on the basis of connectional strength of long-range and short-range connections (see the supplementary material for details of SVM procedure and results). We also compared all the connectivity strength of 4005 pairs among the 3 groups using a Bonferroni-corrected mass univariate approach (independent t test) to localize the pairs that show the most significant group-specific abnormalities. A flowchart of the analytical procedure is shown in the supplementary material.
Results
Anatomical Distance Function
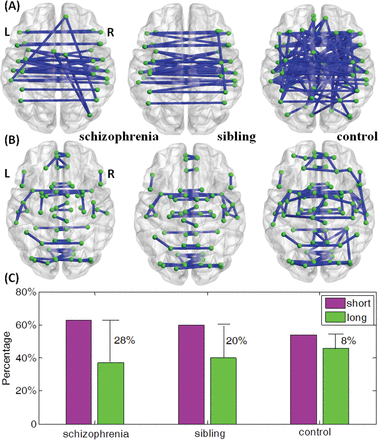
A negative relationship between anatomical distance and connectivity strength was present in all 3 groups, as shown in figure 1C, confirming the expected ADF in adult brains. But ADF was significantly more pronounced in patients with schizophrenia compared with siblings and controls. MAD was significantly shorter in patients compared with controls. S-long was significantly reduced (more pronounced negative correlations) in patients but increased (less pronounced negative correlations) in siblings. S-short was significantly increased (more pronounced positive correlations) in both patients and siblings, with siblings showing greater connectivity strength than patients, as shown in figure 1D. Both siblings and patients showed a reduction in P-long and an increase in P-short. These results are shown in figure 2 and table 2. The result of the SVM analysis is presented in the supplementary material.
Anatomical Distance Indices in the 3 Groups
| . | Scz . | Sib . | Con . | t/χ2and F tests . |
|---|---|---|---|---|
| ADF | −0.5979 (0.0455) | −0.5396 (0.0573) | −0.4381 (0.0530) | ANOVA: F = 98.71, P < .001 Con vs Scz: t = 13.7550, P < .001 Con vs Sib: t = 8.1550, P < .001 Sib vs Scz: t = 4.3170, P < .001 |
| MAD | 34.7333 (28.7373) | 44.3558 (33.1309) | 45.0083 (30.5165) | ANOVA: F = 3.19, P = .043 Con vs Scz: t = 2.5025 P = .0130 Con vs Sib: t = 0.1518, P = 0.8794 Sib vs Scz: t = 1.9377, P = .0545 |
| S-long | −0.063 (0.032) | −0.0187 (0.027) | −0.0562 (0.023) | ANOVA: F = 24.16, P < .001 Con vs Scz: t = 1.1534, P = .2519 Con vs Sib: t = −6.6623, P < .001 Sib vs Scz: t = 5.0871, P < .001 |
| S-short | 0.26 (0.0320) | 0.2795 (0.0450) | 0.1487 (0.0328) | ANOVA: F = 166.79, P < .001 Con vs Scz: t = −14.9515, P < .001 Con vs Sib: t = −15.4335, P < .001 Sib vs Scz: t = 1.8361, P = .0774 |
| P-long vs P-short | 36% vs 64% | 40% vs 60% | 46% vs 54% | Con vs Scz: χ2 = 17.5569, P < .001 Con vs Sib: χ2 = 7.6874, P = .0056 Sib vs Scz: χ2 = 1.5110, P = .2090 |
| . | Scz . | Sib . | Con . | t/χ2and F tests . |
|---|---|---|---|---|
| ADF | −0.5979 (0.0455) | −0.5396 (0.0573) | −0.4381 (0.0530) | ANOVA: F = 98.71, P < .001 Con vs Scz: t = 13.7550, P < .001 Con vs Sib: t = 8.1550, P < .001 Sib vs Scz: t = 4.3170, P < .001 |
| MAD | 34.7333 (28.7373) | 44.3558 (33.1309) | 45.0083 (30.5165) | ANOVA: F = 3.19, P = .043 Con vs Scz: t = 2.5025 P = .0130 Con vs Sib: t = 0.1518, P = 0.8794 Sib vs Scz: t = 1.9377, P = .0545 |
| S-long | −0.063 (0.032) | −0.0187 (0.027) | −0.0562 (0.023) | ANOVA: F = 24.16, P < .001 Con vs Scz: t = 1.1534, P = .2519 Con vs Sib: t = −6.6623, P < .001 Sib vs Scz: t = 5.0871, P < .001 |
| S-short | 0.26 (0.0320) | 0.2795 (0.0450) | 0.1487 (0.0328) | ANOVA: F = 166.79, P < .001 Con vs Scz: t = −14.9515, P < .001 Con vs Sib: t = −15.4335, P < .001 Sib vs Scz: t = 1.8361, P = .0774 |
| P-long vs P-short | 36% vs 64% | 40% vs 60% | 46% vs 54% | Con vs Scz: χ2 = 17.5569, P < .001 Con vs Sib: χ2 = 7.6874, P = .0056 Sib vs Scz: χ2 = 1.5110, P = .2090 |
Note: ADF, anatomical distance function; MAD, mean anatomical distance; S-long, connectivity strength of long-range connections; S-short, connectivity strength of short-range connections; P-long, proportion of all long-range connections; P-short, proportion of all short-range connections; Scz, patients with schizophrenia; Sib, healthy siblings of patients with schizophrenia; Con, healthy controls. Numbers in brackets represent the standard deviation.
Anatomical Distance Indices in the 3 Groups
| . | Scz . | Sib . | Con . | t/χ2and F tests . |
|---|---|---|---|---|
| ADF | −0.5979 (0.0455) | −0.5396 (0.0573) | −0.4381 (0.0530) | ANOVA: F = 98.71, P < .001 Con vs Scz: t = 13.7550, P < .001 Con vs Sib: t = 8.1550, P < .001 Sib vs Scz: t = 4.3170, P < .001 |
| MAD | 34.7333 (28.7373) | 44.3558 (33.1309) | 45.0083 (30.5165) | ANOVA: F = 3.19, P = .043 Con vs Scz: t = 2.5025 P = .0130 Con vs Sib: t = 0.1518, P = 0.8794 Sib vs Scz: t = 1.9377, P = .0545 |
| S-long | −0.063 (0.032) | −0.0187 (0.027) | −0.0562 (0.023) | ANOVA: F = 24.16, P < .001 Con vs Scz: t = 1.1534, P = .2519 Con vs Sib: t = −6.6623, P < .001 Sib vs Scz: t = 5.0871, P < .001 |
| S-short | 0.26 (0.0320) | 0.2795 (0.0450) | 0.1487 (0.0328) | ANOVA: F = 166.79, P < .001 Con vs Scz: t = −14.9515, P < .001 Con vs Sib: t = −15.4335, P < .001 Sib vs Scz: t = 1.8361, P = .0774 |
| P-long vs P-short | 36% vs 64% | 40% vs 60% | 46% vs 54% | Con vs Scz: χ2 = 17.5569, P < .001 Con vs Sib: χ2 = 7.6874, P = .0056 Sib vs Scz: χ2 = 1.5110, P = .2090 |
| . | Scz . | Sib . | Con . | t/χ2and F tests . |
|---|---|---|---|---|
| ADF | −0.5979 (0.0455) | −0.5396 (0.0573) | −0.4381 (0.0530) | ANOVA: F = 98.71, P < .001 Con vs Scz: t = 13.7550, P < .001 Con vs Sib: t = 8.1550, P < .001 Sib vs Scz: t = 4.3170, P < .001 |
| MAD | 34.7333 (28.7373) | 44.3558 (33.1309) | 45.0083 (30.5165) | ANOVA: F = 3.19, P = .043 Con vs Scz: t = 2.5025 P = .0130 Con vs Sib: t = 0.1518, P = 0.8794 Sib vs Scz: t = 1.9377, P = .0545 |
| S-long | −0.063 (0.032) | −0.0187 (0.027) | −0.0562 (0.023) | ANOVA: F = 24.16, P < .001 Con vs Scz: t = 1.1534, P = .2519 Con vs Sib: t = −6.6623, P < .001 Sib vs Scz: t = 5.0871, P < .001 |
| S-short | 0.26 (0.0320) | 0.2795 (0.0450) | 0.1487 (0.0328) | ANOVA: F = 166.79, P < .001 Con vs Scz: t = −14.9515, P < .001 Con vs Sib: t = −15.4335, P < .001 Sib vs Scz: t = 1.8361, P = .0774 |
| P-long vs P-short | 36% vs 64% | 40% vs 60% | 46% vs 54% | Con vs Scz: χ2 = 17.5569, P < .001 Con vs Sib: χ2 = 7.6874, P = .0056 Sib vs Scz: χ2 = 1.5110, P = .2090 |
Note: ADF, anatomical distance function; MAD, mean anatomical distance; S-long, connectivity strength of long-range connections; S-short, connectivity strength of short-range connections; P-long, proportion of all long-range connections; P-short, proportion of all short-range connections; Scz, patients with schizophrenia; Sib, healthy siblings of patients with schizophrenia; Con, healthy controls. Numbers in brackets represent the standard deviation.
We wish to print this figure with color reproduction too. The top 10% of significant long links from 1-sample t tests within each group are displayed in panel (A). There are 29 long links within schizophrenia group, 31 long links within sibling group, and 76 long links within controls. The top 10% of significant short links within each group are displayed in panel (B). There are 49 short links within schizophrenia group, 47 short links within sibling group and 92 short links within controls. (C) Bar plot of the proportional difference of short links and long links within each group displaying the difference between P-short and P-long shown in Supplementary Data.
Frontal, Insular, and Subcortical Systems
ROIs related to frontal network, subcortical network, and salience network are elaborated in supplementary materials. Proportional contribution of frontal regions to the significant short and long-range connections was unaffected in siblings and patients. There was a disproportionate decrease in long-range connections and increase in short-range connections involving subcortical regions in both patients and siblings. An increase in long-range connections involving the salience network was seen in patients. In line with the overall changes in S-long, the frontal, insular, and subcortical long-range connections showed more pronounced negative correlations in patients while the short-range connections showed more pronounced positive correlations. Siblings showed either less pronounced negative correlation (frontal, subcortical) or an unexpected positive correlation (salience network) for long links. These results are shown in table 3.
Regional Changes of Frontal, Subcortical, and Salience Networks
| . | Scz . | Sib . | Con . | t and χ2 tests . |
|---|---|---|---|---|
| Frontal network | ||||
| Proportion of frontal links among all long links | 61% | 60% | 60% | Scz vs Con: χ2 = 0.0156, P = .9007 |
| Sib vs Con: χ2 = 0.0120, P = .8993 | ||||
| Scz vs Sib: χ2 = 0.0438, P = .8342 | ||||
| S-long | −0.1450 (0.0460) | −0.0684 (0.0308) | −0.0805 (0.0297) | ANOVA, F = 42.61, P < .001 |
| Scz vs Con: t = −7.8828, P < .001 | ||||
| Sib vs Con: t = 1.7609, P = .0818 | ||||
| Scz vs Sib: t = −6.4795, P < .001 | ||||
| S-short | 0.2022 (0.0369) | 0.2538 (0.0428) | 0.1140 (0.0343) | ANOVA, F = 150.2, P < .001 |
| Scz vs Con: t = 10.9656, P < .001 | ||||
| Sib vs Con: t = 16.4385, P < .001 | ||||
| Scz vs Sib: t = 4.6471, P < .001 | ||||
| Subcortical network | ||||
| Proportion of subcortical links among all long links | 7% | 7% | 13% | Scz vs Con: χ2 = 8.4627, P = .0036 |
| Sib vs Con: χ2 = 7.7226, P = .0055 | ||||
| Scz vs Sib: χ2 = 0.0477, P = .8270 | ||||
| S-long | −0.2051 (0.0558) | −0.1322 (0.0510) | −0.1135 (0.0514) | ANOVA, F = 29.87, P < .001 |
| Scz vs Con: t = −7.5688, P < .001 | ||||
| Sib vs Con: t = −1.5875, P = .1161 | ||||
| Scz vs Sib: t = −7.9391, P < .001 | ||||
| S-short | 0.2679 (0.0569) | 0.3107 (0.0894) | 0.1738 (0.0602) | ANOVA, F = 45, P < .001 |
| Scz vs Con: t = 6.9424, P < .001 | ||||
| Sib vs Con: t = 8.4614, P < .001 | ||||
| Scz vs Sib: t = −2.1696, P = .0390 | ||||
| SN | ||||
| Proportion of SN links among all long links | 16% | 15% | 12% | Scz vs Con: χ2 = 4.0964, P = .0430 |
| Sib vs Con: χ2 = 1.9501, P = .1626 | ||||
| Scz vs Sib: χ2 = 0.2873, P = .5919 | ||||
| S-long | −0.0670 (0.0426) | 0.0265 (0.0602) | −0.0169 (0.0439) | ANOVA, F = 27.07, P < .001 |
| Scz vs Con: t = −5.0434, P < .001 | ||||
| Sib vs Con: t = 3.8472, P < .001 | ||||
| Scz vs Sib: t = −6.6405, P < .001 | ||||
| S-short | 0.2392 (0.0387) | 0.3265 (0.0813) | 0.2135 (0.0590) | ANOVA, F = 32.8, P < .001 |
| Scz vs Con: t = 2.1036, P = .0383 | ||||
| Sib vs Con: t = 7.3948, P < .001 | ||||
| Scz vs Sib: t = −5.2443, P < .001 | ||||
| . | Scz . | Sib . | Con . | t and χ2 tests . |
|---|---|---|---|---|
| Frontal network | ||||
| Proportion of frontal links among all long links | 61% | 60% | 60% | Scz vs Con: χ2 = 0.0156, P = .9007 |
| Sib vs Con: χ2 = 0.0120, P = .8993 | ||||
| Scz vs Sib: χ2 = 0.0438, P = .8342 | ||||
| S-long | −0.1450 (0.0460) | −0.0684 (0.0308) | −0.0805 (0.0297) | ANOVA, F = 42.61, P < .001 |
| Scz vs Con: t = −7.8828, P < .001 | ||||
| Sib vs Con: t = 1.7609, P = .0818 | ||||
| Scz vs Sib: t = −6.4795, P < .001 | ||||
| S-short | 0.2022 (0.0369) | 0.2538 (0.0428) | 0.1140 (0.0343) | ANOVA, F = 150.2, P < .001 |
| Scz vs Con: t = 10.9656, P < .001 | ||||
| Sib vs Con: t = 16.4385, P < .001 | ||||
| Scz vs Sib: t = 4.6471, P < .001 | ||||
| Subcortical network | ||||
| Proportion of subcortical links among all long links | 7% | 7% | 13% | Scz vs Con: χ2 = 8.4627, P = .0036 |
| Sib vs Con: χ2 = 7.7226, P = .0055 | ||||
| Scz vs Sib: χ2 = 0.0477, P = .8270 | ||||
| S-long | −0.2051 (0.0558) | −0.1322 (0.0510) | −0.1135 (0.0514) | ANOVA, F = 29.87, P < .001 |
| Scz vs Con: t = −7.5688, P < .001 | ||||
| Sib vs Con: t = −1.5875, P = .1161 | ||||
| Scz vs Sib: t = −7.9391, P < .001 | ||||
| S-short | 0.2679 (0.0569) | 0.3107 (0.0894) | 0.1738 (0.0602) | ANOVA, F = 45, P < .001 |
| Scz vs Con: t = 6.9424, P < .001 | ||||
| Sib vs Con: t = 8.4614, P < .001 | ||||
| Scz vs Sib: t = −2.1696, P = .0390 | ||||
| SN | ||||
| Proportion of SN links among all long links | 16% | 15% | 12% | Scz vs Con: χ2 = 4.0964, P = .0430 |
| Sib vs Con: χ2 = 1.9501, P = .1626 | ||||
| Scz vs Sib: χ2 = 0.2873, P = .5919 | ||||
| S-long | −0.0670 (0.0426) | 0.0265 (0.0602) | −0.0169 (0.0439) | ANOVA, F = 27.07, P < .001 |
| Scz vs Con: t = −5.0434, P < .001 | ||||
| Sib vs Con: t = 3.8472, P < .001 | ||||
| Scz vs Sib: t = −6.6405, P < .001 | ||||
| S-short | 0.2392 (0.0387) | 0.3265 (0.0813) | 0.2135 (0.0590) | ANOVA, F = 32.8, P < .001 |
| Scz vs Con: t = 2.1036, P = .0383 | ||||
| Sib vs Con: t = 7.3948, P < .001 | ||||
| Scz vs Sib: t = −5.2443, P < .001 | ||||
Note: SN, salience network; abbreviations are explained in the footnote to table 2.
Regional Changes of Frontal, Subcortical, and Salience Networks
| . | Scz . | Sib . | Con . | t and χ2 tests . |
|---|---|---|---|---|
| Frontal network | ||||
| Proportion of frontal links among all long links | 61% | 60% | 60% | Scz vs Con: χ2 = 0.0156, P = .9007 |
| Sib vs Con: χ2 = 0.0120, P = .8993 | ||||
| Scz vs Sib: χ2 = 0.0438, P = .8342 | ||||
| S-long | −0.1450 (0.0460) | −0.0684 (0.0308) | −0.0805 (0.0297) | ANOVA, F = 42.61, P < .001 |
| Scz vs Con: t = −7.8828, P < .001 | ||||
| Sib vs Con: t = 1.7609, P = .0818 | ||||
| Scz vs Sib: t = −6.4795, P < .001 | ||||
| S-short | 0.2022 (0.0369) | 0.2538 (0.0428) | 0.1140 (0.0343) | ANOVA, F = 150.2, P < .001 |
| Scz vs Con: t = 10.9656, P < .001 | ||||
| Sib vs Con: t = 16.4385, P < .001 | ||||
| Scz vs Sib: t = 4.6471, P < .001 | ||||
| Subcortical network | ||||
| Proportion of subcortical links among all long links | 7% | 7% | 13% | Scz vs Con: χ2 = 8.4627, P = .0036 |
| Sib vs Con: χ2 = 7.7226, P = .0055 | ||||
| Scz vs Sib: χ2 = 0.0477, P = .8270 | ||||
| S-long | −0.2051 (0.0558) | −0.1322 (0.0510) | −0.1135 (0.0514) | ANOVA, F = 29.87, P < .001 |
| Scz vs Con: t = −7.5688, P < .001 | ||||
| Sib vs Con: t = −1.5875, P = .1161 | ||||
| Scz vs Sib: t = −7.9391, P < .001 | ||||
| S-short | 0.2679 (0.0569) | 0.3107 (0.0894) | 0.1738 (0.0602) | ANOVA, F = 45, P < .001 |
| Scz vs Con: t = 6.9424, P < .001 | ||||
| Sib vs Con: t = 8.4614, P < .001 | ||||
| Scz vs Sib: t = −2.1696, P = .0390 | ||||
| SN | ||||
| Proportion of SN links among all long links | 16% | 15% | 12% | Scz vs Con: χ2 = 4.0964, P = .0430 |
| Sib vs Con: χ2 = 1.9501, P = .1626 | ||||
| Scz vs Sib: χ2 = 0.2873, P = .5919 | ||||
| S-long | −0.0670 (0.0426) | 0.0265 (0.0602) | −0.0169 (0.0439) | ANOVA, F = 27.07, P < .001 |
| Scz vs Con: t = −5.0434, P < .001 | ||||
| Sib vs Con: t = 3.8472, P < .001 | ||||
| Scz vs Sib: t = −6.6405, P < .001 | ||||
| S-short | 0.2392 (0.0387) | 0.3265 (0.0813) | 0.2135 (0.0590) | ANOVA, F = 32.8, P < .001 |
| Scz vs Con: t = 2.1036, P = .0383 | ||||
| Sib vs Con: t = 7.3948, P < .001 | ||||
| Scz vs Sib: t = −5.2443, P < .001 | ||||
| . | Scz . | Sib . | Con . | t and χ2 tests . |
|---|---|---|---|---|
| Frontal network | ||||
| Proportion of frontal links among all long links | 61% | 60% | 60% | Scz vs Con: χ2 = 0.0156, P = .9007 |
| Sib vs Con: χ2 = 0.0120, P = .8993 | ||||
| Scz vs Sib: χ2 = 0.0438, P = .8342 | ||||
| S-long | −0.1450 (0.0460) | −0.0684 (0.0308) | −0.0805 (0.0297) | ANOVA, F = 42.61, P < .001 |
| Scz vs Con: t = −7.8828, P < .001 | ||||
| Sib vs Con: t = 1.7609, P = .0818 | ||||
| Scz vs Sib: t = −6.4795, P < .001 | ||||
| S-short | 0.2022 (0.0369) | 0.2538 (0.0428) | 0.1140 (0.0343) | ANOVA, F = 150.2, P < .001 |
| Scz vs Con: t = 10.9656, P < .001 | ||||
| Sib vs Con: t = 16.4385, P < .001 | ||||
| Scz vs Sib: t = 4.6471, P < .001 | ||||
| Subcortical network | ||||
| Proportion of subcortical links among all long links | 7% | 7% | 13% | Scz vs Con: χ2 = 8.4627, P = .0036 |
| Sib vs Con: χ2 = 7.7226, P = .0055 | ||||
| Scz vs Sib: χ2 = 0.0477, P = .8270 | ||||
| S-long | −0.2051 (0.0558) | −0.1322 (0.0510) | −0.1135 (0.0514) | ANOVA, F = 29.87, P < .001 |
| Scz vs Con: t = −7.5688, P < .001 | ||||
| Sib vs Con: t = −1.5875, P = .1161 | ||||
| Scz vs Sib: t = −7.9391, P < .001 | ||||
| S-short | 0.2679 (0.0569) | 0.3107 (0.0894) | 0.1738 (0.0602) | ANOVA, F = 45, P < .001 |
| Scz vs Con: t = 6.9424, P < .001 | ||||
| Sib vs Con: t = 8.4614, P < .001 | ||||
| Scz vs Sib: t = −2.1696, P = .0390 | ||||
| SN | ||||
| Proportion of SN links among all long links | 16% | 15% | 12% | Scz vs Con: χ2 = 4.0964, P = .0430 |
| Sib vs Con: χ2 = 1.9501, P = .1626 | ||||
| Scz vs Sib: χ2 = 0.2873, P = .5919 | ||||
| S-long | −0.0670 (0.0426) | 0.0265 (0.0602) | −0.0169 (0.0439) | ANOVA, F = 27.07, P < .001 |
| Scz vs Con: t = −5.0434, P < .001 | ||||
| Sib vs Con: t = 3.8472, P < .001 | ||||
| Scz vs Sib: t = −6.6405, P < .001 | ||||
| S-short | 0.2392 (0.0387) | 0.3265 (0.0813) | 0.2135 (0.0590) | ANOVA, F = 32.8, P < .001 |
| Scz vs Con: t = 2.1036, P = .0383 | ||||
| Sib vs Con: t = 7.3948, P < .001 | ||||
| Scz vs Sib: t = −5.2443, P < .001 | ||||
Note: SN, salience network; abbreviations are explained in the footnote to table 2.
Interhemispheric Links
The proportion of interhemispheric links among all connections was comparable among the groups (49% in controls; 48% in patients, and 51% in siblings). But when the proportion of long-range connections among all interhemispheric connections were considered, 62% of all interhemispheric connections were long range for controls; only 52% were long range in schizophrenia, with 58% being long range in siblings. The mean connectivity strength for interhemispheric links (both short and long-range) was significantly increased (more pronounced positive correlation) in siblings (mean [SD] = 0.141 [0.033]) and patients (mean [SD] = 0.125 [0.037]) when compared with controls (mean [SD] = 0.044 [0.021]) (ANOVA, F = 141.56, P ≤ .001; patients vs controls, t = 12.93, P < .001; siblings vs controls, t = 16.64, P < .001; patients vs siblings, t = −1.74, P = .093).
Localizing Altered Connectivity
Figure S4 (Supplementary Material) is the Manhattan plot of P value for all of the possible links, where y-axis is 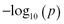 for visualization. On comparison, 15 links emerged as significant for schizophrenia vs controls, while 4 were significant for siblings vs controls. Only 2 pairs of connections (both involving the insula) emerged as significant when patients were compared with their siblings. These results are shown in table 4 and supplementary figure S4.
for visualization. On comparison, 15 links emerged as significant for schizophrenia vs controls, while 4 were significant for siblings vs controls. Only 2 pairs of connections (both involving the insula) emerged as significant when patients were compared with their siblings. These results are shown in table 4 and supplementary figure S4.
The Links With Significant Difference Between the Groups After Bonferroni Correction
| (1) Patients With Schizophrenia vs Healthy Controls . | |||||
|---|---|---|---|---|---|
| Links . | Connectivity strength in Scz . | Connectivity strength in Con . | P value (×10–4) . | Distance (mm) . | |
| 1 | SMG.L−IFGtriang.R | 0.0590 | 0.3132 | .0005 | 124.8877 |
| 2 | TPOmid.R−FFG.R | 0.0129 | 0.2471 | .0096 | 55.9338 |
| 3 | PCUN.L−SFGmed.R | −0.0597 | 0.2539 | .0118 | 109.6049 |
| 4 | SMG.L−IFGoperc.R | 0.1267 | 0.4011 | .0154 | 116.9594 |
| 5 | ITG.R−MTG.R | 0.4214 | 0.1612 | .0187 | 22.0671 |
| 6 | MTG.L−CAL.L | −0.2171 | 0.0816 | .0201 | 66.5477 |
| 7 | TPOsup.R−FFG.R | −0.1440 | 0.0712 | .0202 | 55.8101 |
| 8 | TPOmid.R−IFGtriang.R | 0.0992 | −0.1339 | .0414 | 49.3352 |
| 9 | INS.L−ORBinf.R | 0.0286 | 0.2819 | .0480 | 81.9712 |
| 10 | TPOsup.L−MOG.R | −0.1717 | 0.0542 | .0569 | 128.5824 |
| 11 | SFGdor.R−SPG.L | −0.2539 | 0.0490 | .0580 | 102.5120 |
| 12 | SFGdor.R−IPL.L | −0.2361 | 0.0409 | .0708 | 100.5703 |
| 13 | SFGdor.R−ORBinf.R | 0.1041 | −0.1389 | .0833 | 58.9943 |
| 14 | PUT.R−INS.L | 0.2892 | 0.4757 | .1069 | 62.9417 |
| 15 | ITG.L−PCUN.R | 0.0539 | −0.1630 | .1103 | 93.9948 |
| (2) Siblings vs Healthy Controls | |||||
| Links | Connectivity strength in Sib | Connectivity strength in Con | P value (×10–4) | Distance (mm) | |
| 1 | ANG.L−SPG.R | 0.0248 | −0.2638 | .0000 | 75.0894 |
| 2 | PCUN.L−SFGmed.R | −0.0475 | 0.2539 | .0417 | 109.6049 |
| 3 | AMYG.L−SMA.L | 0.0984 0.0984 | −0.1282 | .1080 | 80.7345 |
| 4 | TPOsup.L−MOG.R | −0.1550 | 0.0542 | .1220 | 128.5824 |
| (3) Patients With Schizophrenia vs Siblings | |||||
| Links | Connectivity strength in Scz | Connectivity strength in Sib | P value (×10–4) | Distance (mm) | |
| 1 | INS.L−REC.R | −0.3176 | −0.0286 | .0108 | 56.5006 |
| 2 | INS.R−REC.R | −0.3175 | −0.0157 | .0270 | 47.0025 |
| (1) Patients With Schizophrenia vs Healthy Controls . | |||||
|---|---|---|---|---|---|
| Links . | Connectivity strength in Scz . | Connectivity strength in Con . | P value (×10–4) . | Distance (mm) . | |
| 1 | SMG.L−IFGtriang.R | 0.0590 | 0.3132 | .0005 | 124.8877 |
| 2 | TPOmid.R−FFG.R | 0.0129 | 0.2471 | .0096 | 55.9338 |
| 3 | PCUN.L−SFGmed.R | −0.0597 | 0.2539 | .0118 | 109.6049 |
| 4 | SMG.L−IFGoperc.R | 0.1267 | 0.4011 | .0154 | 116.9594 |
| 5 | ITG.R−MTG.R | 0.4214 | 0.1612 | .0187 | 22.0671 |
| 6 | MTG.L−CAL.L | −0.2171 | 0.0816 | .0201 | 66.5477 |
| 7 | TPOsup.R−FFG.R | −0.1440 | 0.0712 | .0202 | 55.8101 |
| 8 | TPOmid.R−IFGtriang.R | 0.0992 | −0.1339 | .0414 | 49.3352 |
| 9 | INS.L−ORBinf.R | 0.0286 | 0.2819 | .0480 | 81.9712 |
| 10 | TPOsup.L−MOG.R | −0.1717 | 0.0542 | .0569 | 128.5824 |
| 11 | SFGdor.R−SPG.L | −0.2539 | 0.0490 | .0580 | 102.5120 |
| 12 | SFGdor.R−IPL.L | −0.2361 | 0.0409 | .0708 | 100.5703 |
| 13 | SFGdor.R−ORBinf.R | 0.1041 | −0.1389 | .0833 | 58.9943 |
| 14 | PUT.R−INS.L | 0.2892 | 0.4757 | .1069 | 62.9417 |
| 15 | ITG.L−PCUN.R | 0.0539 | −0.1630 | .1103 | 93.9948 |
| (2) Siblings vs Healthy Controls | |||||
| Links | Connectivity strength in Sib | Connectivity strength in Con | P value (×10–4) | Distance (mm) | |
| 1 | ANG.L−SPG.R | 0.0248 | −0.2638 | .0000 | 75.0894 |
| 2 | PCUN.L−SFGmed.R | −0.0475 | 0.2539 | .0417 | 109.6049 |
| 3 | AMYG.L−SMA.L | 0.0984 0.0984 | −0.1282 | .1080 | 80.7345 |
| 4 | TPOsup.L−MOG.R | −0.1550 | 0.0542 | .1220 | 128.5824 |
| (3) Patients With Schizophrenia vs Siblings | |||||
| Links | Connectivity strength in Scz | Connectivity strength in Sib | P value (×10–4) | Distance (mm) | |
| 1 | INS.L−REC.R | −0.3176 | −0.0286 | .0108 | 56.5006 |
| 2 | INS.R−REC.R | −0.3175 | −0.0157 | .0270 | 47.0025 |
Note: Scz, patients with schizophrenia; Sib, siblings, Con, controls. The descriptors for regions-of-interest are provided in the Supplementary Material.
The Links With Significant Difference Between the Groups After Bonferroni Correction
| (1) Patients With Schizophrenia vs Healthy Controls . | |||||
|---|---|---|---|---|---|
| Links . | Connectivity strength in Scz . | Connectivity strength in Con . | P value (×10–4) . | Distance (mm) . | |
| 1 | SMG.L−IFGtriang.R | 0.0590 | 0.3132 | .0005 | 124.8877 |
| 2 | TPOmid.R−FFG.R | 0.0129 | 0.2471 | .0096 | 55.9338 |
| 3 | PCUN.L−SFGmed.R | −0.0597 | 0.2539 | .0118 | 109.6049 |
| 4 | SMG.L−IFGoperc.R | 0.1267 | 0.4011 | .0154 | 116.9594 |
| 5 | ITG.R−MTG.R | 0.4214 | 0.1612 | .0187 | 22.0671 |
| 6 | MTG.L−CAL.L | −0.2171 | 0.0816 | .0201 | 66.5477 |
| 7 | TPOsup.R−FFG.R | −0.1440 | 0.0712 | .0202 | 55.8101 |
| 8 | TPOmid.R−IFGtriang.R | 0.0992 | −0.1339 | .0414 | 49.3352 |
| 9 | INS.L−ORBinf.R | 0.0286 | 0.2819 | .0480 | 81.9712 |
| 10 | TPOsup.L−MOG.R | −0.1717 | 0.0542 | .0569 | 128.5824 |
| 11 | SFGdor.R−SPG.L | −0.2539 | 0.0490 | .0580 | 102.5120 |
| 12 | SFGdor.R−IPL.L | −0.2361 | 0.0409 | .0708 | 100.5703 |
| 13 | SFGdor.R−ORBinf.R | 0.1041 | −0.1389 | .0833 | 58.9943 |
| 14 | PUT.R−INS.L | 0.2892 | 0.4757 | .1069 | 62.9417 |
| 15 | ITG.L−PCUN.R | 0.0539 | −0.1630 | .1103 | 93.9948 |
| (2) Siblings vs Healthy Controls | |||||
| Links | Connectivity strength in Sib | Connectivity strength in Con | P value (×10–4) | Distance (mm) | |
| 1 | ANG.L−SPG.R | 0.0248 | −0.2638 | .0000 | 75.0894 |
| 2 | PCUN.L−SFGmed.R | −0.0475 | 0.2539 | .0417 | 109.6049 |
| 3 | AMYG.L−SMA.L | 0.0984 0.0984 | −0.1282 | .1080 | 80.7345 |
| 4 | TPOsup.L−MOG.R | −0.1550 | 0.0542 | .1220 | 128.5824 |
| (3) Patients With Schizophrenia vs Siblings | |||||
| Links | Connectivity strength in Scz | Connectivity strength in Sib | P value (×10–4) | Distance (mm) | |
| 1 | INS.L−REC.R | −0.3176 | −0.0286 | .0108 | 56.5006 |
| 2 | INS.R−REC.R | −0.3175 | −0.0157 | .0270 | 47.0025 |
| (1) Patients With Schizophrenia vs Healthy Controls . | |||||
|---|---|---|---|---|---|
| Links . | Connectivity strength in Scz . | Connectivity strength in Con . | P value (×10–4) . | Distance (mm) . | |
| 1 | SMG.L−IFGtriang.R | 0.0590 | 0.3132 | .0005 | 124.8877 |
| 2 | TPOmid.R−FFG.R | 0.0129 | 0.2471 | .0096 | 55.9338 |
| 3 | PCUN.L−SFGmed.R | −0.0597 | 0.2539 | .0118 | 109.6049 |
| 4 | SMG.L−IFGoperc.R | 0.1267 | 0.4011 | .0154 | 116.9594 |
| 5 | ITG.R−MTG.R | 0.4214 | 0.1612 | .0187 | 22.0671 |
| 6 | MTG.L−CAL.L | −0.2171 | 0.0816 | .0201 | 66.5477 |
| 7 | TPOsup.R−FFG.R | −0.1440 | 0.0712 | .0202 | 55.8101 |
| 8 | TPOmid.R−IFGtriang.R | 0.0992 | −0.1339 | .0414 | 49.3352 |
| 9 | INS.L−ORBinf.R | 0.0286 | 0.2819 | .0480 | 81.9712 |
| 10 | TPOsup.L−MOG.R | −0.1717 | 0.0542 | .0569 | 128.5824 |
| 11 | SFGdor.R−SPG.L | −0.2539 | 0.0490 | .0580 | 102.5120 |
| 12 | SFGdor.R−IPL.L | −0.2361 | 0.0409 | .0708 | 100.5703 |
| 13 | SFGdor.R−ORBinf.R | 0.1041 | −0.1389 | .0833 | 58.9943 |
| 14 | PUT.R−INS.L | 0.2892 | 0.4757 | .1069 | 62.9417 |
| 15 | ITG.L−PCUN.R | 0.0539 | −0.1630 | .1103 | 93.9948 |
| (2) Siblings vs Healthy Controls | |||||
| Links | Connectivity strength in Sib | Connectivity strength in Con | P value (×10–4) | Distance (mm) | |
| 1 | ANG.L−SPG.R | 0.0248 | −0.2638 | .0000 | 75.0894 |
| 2 | PCUN.L−SFGmed.R | −0.0475 | 0.2539 | .0417 | 109.6049 |
| 3 | AMYG.L−SMA.L | 0.0984 0.0984 | −0.1282 | .1080 | 80.7345 |
| 4 | TPOsup.L−MOG.R | −0.1550 | 0.0542 | .1220 | 128.5824 |
| (3) Patients With Schizophrenia vs Siblings | |||||
| Links | Connectivity strength in Scz | Connectivity strength in Sib | P value (×10–4) | Distance (mm) | |
| 1 | INS.L−REC.R | −0.3176 | −0.0286 | .0108 | 56.5006 |
| 2 | INS.R−REC.R | −0.3175 | −0.0157 | .0270 | 47.0025 |
Note: Scz, patients with schizophrenia; Sib, siblings, Con, controls. The descriptors for regions-of-interest are provided in the Supplementary Material.
Discussion
Using task-free fMRI we have shown that the ADF is disturbed in adult patients with schizophrenia and their age-matched siblings. This observation suggests that an expected developmental transition in connectivity that is noted in healthy adolescents is likely to be disturbed in relation to the genetic risk of schizophrenia. Both patients and siblings showed a reduction in the proportion of significant long-range connections. While the patients showed a higher degree of anticorrelation in long-range connections and higher degree of positive correlation in short-range connections, siblings demonstrated a shift towards more positive correlation in both short-range and long-range connections. Patients also showed a significant reduction in the mean anatomical distance of significant connections.
The preferential reduction in long-range connections in schizophrenia raises important questions. Numerous observations have suggested that the metabolic and wiring cost of long-distance connections are in general unfavorable to the “low-maintenance” principle of the human brain.39,40 As a result, it has been argued that a drive towards cost efficiency is evolutionarily favored,41 genetically influenced,42 and built into the normal cortical development (ontogenesis).43 But such reduction reaches a tipping point during adolescence, where selective long-distance connections are “nurtured,” leading to the adult brain becoming an efficient hub of information transfer.44 The preferential reduction in long-range connections in patients suggests that either this developmental shift does not take place in patients, or is further delayed into later adulthood. As a result, the efficiency of information transfer in the brain networks of patients with schizophrenia is likely to be affected (see supplementary material). Numerous observations using structural, functional, and electrophysiological studies in schizophrenia indeed demonstrate evidence for such reduced efficiency. Further, there is some evidence to suggest that the architecture of functional brain networks in schizophrenia confer a degree of advantage (“robustness”) to the connected system,45 which in turn may explain the failure of the forces of natural selection to reduce the incidence of schizophrenia.
The local to distributed change in connectivity seen in adolescence appears important for the interactive specialization of the brain regions in adults.44 According to this notion, at birth, cortical regions are broadly tuned and less selective with their connectivity constrained by anatomical distance and connectivity; with age, simultaneous segregation and integration takes place.46 In this context, patients with schizophrenia are likely to show a failure of interactive specialization. As a result, when cognitive demands are elevated, “non-specialized” cortical regions must be recruited in service of the task, resulting in inefficient but excessive recruitment. Several fMRI studies of task performance in patients support this notion.47–49
We observed that in siblings, though the ADF is perturbed, there is some degree of recovery or compensation. Though long links are reduced in proportion, there is a degree of strengthening of relationship among the existing links, which is not seen in patients. This observation is strikingly reminiscent of the structural studies that demonstrate “normalization” of deficits along the developmental trajectory in siblings.1 While higher genetic loading in patients could be invoked to explain the lack of such “recovery,” another possibility is the effect of environmental agents such as cannabis and stimulants. Exposure to cannabis at developmentally critical time windows appears to have a selective effect on the integrity of structural connections.50 Direct intracortical animal studies suggest that ketamine, which has a propensity to trigger psychotic symptoms, induces decoupling of long-range connections—a finding analogous to the observations made in the present study in patients with schizophrenia.51
Alexander-Bloch et al.34 investigated the relationship between anatomical distance and functional connectivity strength using a graph-based approach in childhood-onset schizophrenia (mean [SD] age at scan = 18.7 [4.9]; mean [SD] age of onset = 10 [1.8]). In line with our results, stronger connections were noted at shorter anatomical distances in both controls and patients. Further, the mean connection distance in sparsely thresholded networks from healthy participants (44 mm) was strikingly similar to the value obtained in our study (45 mm). Despite these similarities, COS group had relatively normal functional connectivity when long-distance links were considered. The greatest disturbance in connectivity in COS was noted for short-distance connections. In contrast to the approach used in our study, Alexander-Bloch et al. considered only absolute values of functional connectivity (ie, negative correlations were treated as positive correlations) and the connectivity matrices were binarized to derive topological metrics from 293 brain regions, in contrast to the 90 used in our study. These methodological differences preclude meaningful comparison of the 2 results. But if a true difference exists in the direction of relationship between anatomical distance and connectivity between COS and adult-onset schizophrenia, then the ADF could be considered as an important biological variable potentially influencing the age of onset of schizophrenia.
The subcortical system showed a disproportionate reduction in the number of long-range connections and an increase in short-range connections in schizophrenia. An increase in short-range connectivity involving subcortical structures in schizophrenia has been well documented previously.52,53 There is also a selective reduction of interhemispheric long links in schizophrenia, though when short-range connections are also taken into account, the overall strength of connections shifts towards higher positive correlation in patients. These observations reconcile previously reported inconsistent evidence regarding the increase54,55 or decrease19,20 in interhemispheric transfer, highlighting the importance of exploring anatomical distance while studying functional connectivity in schizophrenia.
Two long-range interhemispheric connections (left precuneus with right medial superior frontal and left temporal pole to right middle occipital gyrus) showed common abnormality in patients and siblings. Siblings showed an unexpected positive correlation in the long-range connections involving the salience network nodes, while patients showed more pronounced anticorrelation. The localization of altered connectivity changes revealed that indeed the greatest difference between siblings and patients related to the connectivity between insula and a portion of the orbitofrontal cortex (rectus gyrus). These observations are interesting in the context of structural studies, which show that the anatomical changes pertaining to the insula is only apparent in patients and not siblings.56 Several lines of evidence now point towards abnormalities in the salience-processing system as a cardinal feature of several core psychotic symptoms.17 The present results reaffirm this notion and additionally suggest that genetically high-risk siblings, who do not have clinical psychotic illness, may have a selective strengthening of the long-range connections involving the salience network, which confers them with a protective effect. In fact, the establishment of structural and dynamic causal connectivity between insula and rest of the brain appears to be a crucial maturational event during adolescence.57 Despite sharing the ADF abnormality with patients, siblings show an excessive strengthening of the salience network–related long-range connections. This phenomenon calls for further investigation of the developmental cortical maturation in siblings using a longitudinal design. If a critical difference in the salience network connectivity is indeed the step change between resilience and psychosis in genetically predisposed individuals, then modulating the salience-processing networks may offer therapeutic opportunities in psychosis.
Several limitations must be borne in mind while interpreting this study. We approached the issue of connectivity using arbitrary anatomical parcellations. This approach is not unconventional; the atlas-based parcellation scheme may facilitate replication of the current work with other data sets. Nevertheless, the functional correspondence of AAL regions is unclear at present. In line with a number of other studies, we used a correlation-based approach to infer brain connectivity, but we did not measure other graph metrics. While the study of topological properties of functional networks is a very interesting area,58,59 our primary interest was the relationship between anatomical distance and the strength of interregional resting-state correlations. Our approach allowed a direct test of our hypothesis, without requiring other topological metrics. Seventy-five percent of our patients were medicated. Antipsychotic medications may attenuate functional connectivity patterns in the short term,60,61 though to our knowledge, there is no evidence to suggest that the ADF is altered by the use of antipsychotics. The absence of experimental evidence to support or refute the effect of antipsychotics on ADF calls for cautious interpretation of our results. We have addressed this issue further in the supplementary material.
In summary, the relationship between anatomical distance and functional connectivity is significantly altered in schizophrenia. The observed abnormalities suggest that the normal adolescent maturational process goes awry in those with a genetic diathesis to develop psychosis, but siblings who are “resilient” to the clinically expressed illness show a degree of normalization or “protective” changes that are absent in patients. Our findings offer a converging framework to examine the effects of genetic risk factors for psychosis on the developing brain. They also raise the question whether the disturbances in the organizing principles of brain connectivity seen in schizophrenia may confer some degree of genetically determined metabolic cost advantage.
Funding
National Centre for Mathematics and Interdisciplinary Sciences of the Chinese Academy of Sciences and Key Program of National Natural Science Foundation of China (91230201 to J.F., Royal Society Wolfson Research Merit Award holder); National Natural Science Foundation of China (11271121 to S.G.); Program for New Century Excellent Talents in University (NCET-13-0786 to S.G.), Key Laboratory of Computational and Stochastic Mathematics and Its Application of Hunan Province (11K038 to S.G.) and the Construct Program of the Key Discipline in Hunan Province; Wellcome Trust (Research Training Fellowship [WT096002/Z/11] to L.P.).
Acknowledgments
Dr Yang, Dr Liu, and Dr Xue collected the data from Xiangya hospital, and they had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Dr Guo, Dr Palaniyappan, and Dr Feng analyzed and interpreted the data and wrote the article. LP acknowledges receiving a travel fellowship to attend Ninth Bipolar Disorder Conference, supported by Eli Lilly in 2010. All other authors reported no biomedical financial interests or potential conflicts of interest.
References